
11 May Interleukin-1 blockade with high-dose Anakinra in patients with COVID-19
‘The Lancet Rheumatology’ has published an important treatment success using high-dose Anakinra, which is biological disease modifying anti-rheumatic drug (biological DMARD).
This retrospective cohort study of patients with COVID-19 and acute respiratory distress syndrome (ARDS) managed with non-invasive ventilation outside of ICU and treated with anakinra was associated with clinical improvement in 72% of patients. High -dose intravenous anakinra dampened systemic inflammation and was associated with progressive respiratory function improvement which allowed doctors to either postpone of avoid intubation in most patients. Furthermore, the treatment was well tolerated and discontinuation was not followed by relapses in systemic inflammation or respiratory dysfunction.
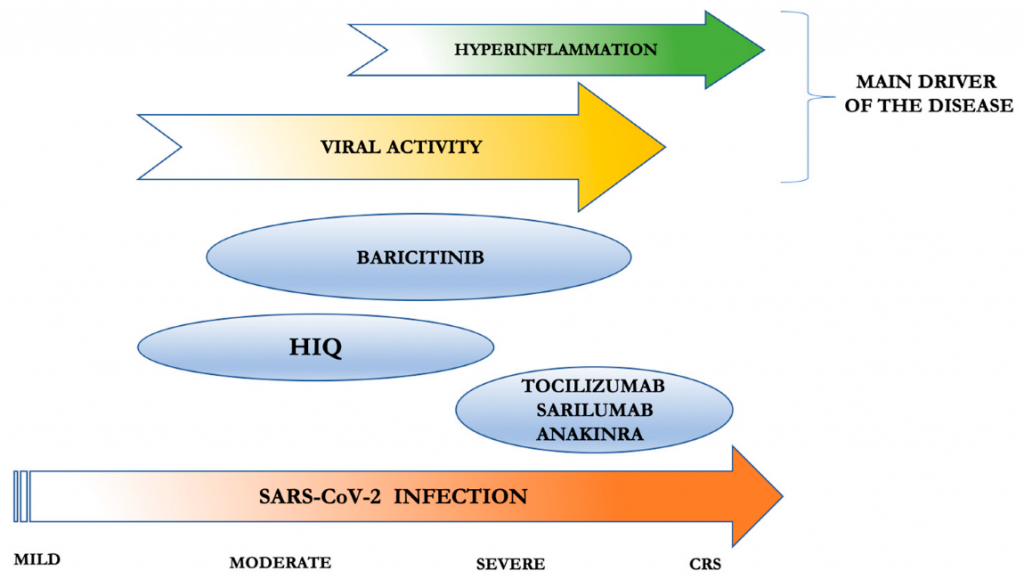
Figure. Anakinra use in COVID-19 treatment
In view of the urgent need for an effective treatment for COVID-19, patients world-wide have received several off-label treatments or compassionate-use treatments. The severity of patients in this study was indeed notable and whilst the studies authors acknowledge clear limitations, the results provide a further advancement in the hope of developing a standardised framework in treatment for COVID-19.
For further information regarding Anakinra please refer to:
https://www.nps.org.au/australian-prescriber/articles/anakinra
PulmCrit – High-dose anakinra for COVID-19: The anti-inflammatory trials begin
Source: https://www.thelancet.com/pdfs/journals/lanrhe/PIIS2665-9913(20)30127-2.pdf

