
12 Mar Measuring Blood Gasses
A Blood gas test measures the amount of oxygen, carbon dioxide and pH levels in the blood. Arterial blood gasses or ABGs, are among the more complex assessments performed by a clinical healthcare professional. Imbalances in the oxygen, carbon dioxide and pH levels indicate the presence of a medical condition, it is thus important to be able to identify normal and abnormal parameters of blood gasses.
When analysing and interpreting ABGs it is important to know the ‘normal’ or baseline, from there you can identify variations in the patient’s results which could indicate clinical deterioration.
Indications for ABGs are
- Diagnosis of acute respiratory failure Type I or Type II
- Assess/monitor/base status eg Ketoacidosis
- Manage artificial ventilation
- Check the response to therapy
Steps for interpreting ABGs are as follows:
- Know the normal values
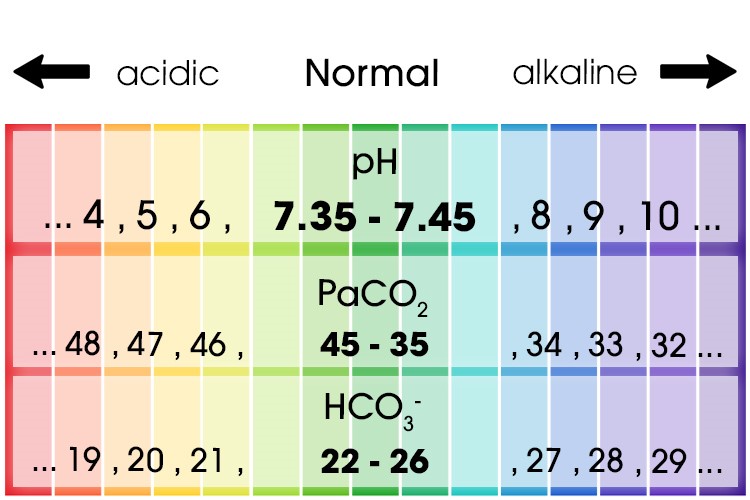
- pH measures the amount of hydrogen ions (H+) in the blood sample. This determines if the blood is acidotic or alkalotic. Normal values for pH is 7.35-7.45
- The next value is the carbon dioxide level, and this will tell you if the problem is respiratory in origin as in CO2 related to the lungs. The normal range for PaCO2 is 35-45mmHg
- The next step is the bicarbonate ions (HCO3), this will tell you if the problem is metabolic and refers to the renal system. Normal is 22-26mmol/L
Normal ABG Levels
| pH | Hydrogen | 7.35 – 7.45 |
| PaCO2 | Carbon dioxide | 35 – 45 mmHg |
| HCO3- | Bicarbonate | 22 – 26 mmol/L |
- pH: Acidosis /Alkalosis
If the pH is not in the normal range the patient is either acidotic or alkalotic

- The lower the number the more acidotic and the higher the number the more base is in the blood sample
- Once pH has been determined, the cause can be looked at
(Kaufman 2020)
- HCO3: Metabolic or Respiratory
- The next step is to find out if the cause is metabolic or respiratory
- If the cause is respiratory in nature, the PaCO2 would be out of the normal range, whereas if it was metabolic in nature the HCO3 would be abnormal. Low PaCO2 points to respiratory alkalosis and high HCO3 indicates metabolic alkalosis
- PaCO2 : Compensated or Uncompensated
- Compensation is the body’s attempt to correct the imbalances. Is one system trying to compensate for an abnormality in another system?
- We investigate this by looking at the opposing component to the problem, for example in acidosis we look at HCO3, in an alkalosis, to determine if the body is compensating, we look at the PaCO2
- If the other level or component is within normal limits, then the problem is non-compensated, or in other words the body has yet to fix the problem or is unable to fix the problem.
- However, if the other component is outside or normal ranges, compensation is occurring (the body is trying to fix the problem). To assess how well it has been able to compensate, we look at the pH, if the pH is close to normal limits the we have partial-compensation, if the pH is back in normal limits we have full-compensation
To simplify:
| Compensated or Uncompensated? |
Respiratory or Metabolic? |
Acidic or Alkalotic? |
pH | PaCO2 | HCO3- |
| Respiratory | Acidosis | Low | High | ||
| Respiratory | Alkalosis | High | Low | ||
| Metabolic | Acidosis | Low | Low | ||
| Metabolic | Alkalosis | High | High | ||
| Compensated | Respiratory | Acidosis | Normal | High | |
| Compensated | Respiratory | Alkalosis | Normal | Low | |
| Compensated | Metabolic | Acidosis | Normal | Low | |
| Compensated | Metabolic | Alkalosis | Normal | High |
Kaufman, DA 2020, Interpretation of ABGs, American Thoracic Society, viewed 20 April 2020, https://www.thoracic.org/professionals/clinical-resources/critical-care/clinical-education/abgs.php

