24 Jun Inhaled protein/peptide-based therapies for respiratory disease
Asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF) are all chronic pulmonary diseases, albeit with different aetiologies, that are characterized by airflow limitation, chronic inflammation, and abnormal mucus production/rheology. Small synthetic molecule-based therapies are commonly prescribed for all three diseases. However, there has been increased interest in “biologicals” to treat these diseases. Biologicals typically constitute protein- or peptide-based therapies and are often more potent than small molecule-based drugs. The lungs allow for efficient drug delivery as they have a large surface area and are well vascularized. The majority of drugs in use today are classed as “small molecules.” That is, organic chemicals typically bind to their receptor to elicit a response. Since these molecules are often extremely durable, until metabolized by the liver and/or cleared by the kidney, they can have side effects in other organs. In contrast, biological therapeutics, including proteins (e.g., antibodies, enzymes) and peptides, show considerable promise and are emerging as alternatives to small molecule-based drugs.
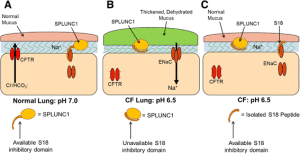
Short palate lung and nasal epithelium clone 1 (SPLUNC1) is a ~25-kDa protein that contains an ENaC inhibitory domain, which for historical reasons was called the S18 region. Unlike traditional ion channel antagonists which block ENaC’s pore, SPLUNC1 inhibits ENaC by inducing endocytosis (Fig. 1a). Since SPLUNC1 fails to regulate ENaC in the CF lung (Fig. 1b), Spyryx Biosciences is currently developing a SPLUNC1-derived peptide, which functions in CF airways as an ENaC inhibitor (Fig. 1c). This restoration of CF airway surface liquid (ASL) hydration is predicted to (i) improve mucociliary clearance and (ii) decrease infection/inflammation. Additionally, these peptides are intrinsically disordered so they are heat stable. Another advantage of intrinsically disordered proteins/peptides is that they achieve a greater contact area with their target protein, thus maximizing binding efficiency. S18-derived peptides are protease resistant, do not freely cross the respiratory epithelium, and do not reach the kidney to induce the hyperkalemia, as seen with small molecule ENaC antagonists like amiloride. Chronic inhalation therapy using these peptides could produce local immunogenicity and irritation, but given that SPLUNC1-derived peptides are naturally occurring in normal but not CF lungs, immunogenicity would seem unlikely. A limitation of this type of therapeutic is that it would only ameliorate CF lung disease and would not treat other CF-affected organs.
Fig. 1
Rationale for SPLUNC1-derived peptide therapy for CF lung disease. a In normal airways, bicarbonate secretion through CFTR maintains ASL pH at ~7.0. At this pH, secreted SPLUNC1 can bind to ENaC, leading to internalization and inhibition of the channel. This helps maintain airways hydration and mucus clearance. b In CF airways, the acidic ASL, caused by a lack of bicarbonate secretion through dysfunctional CFTR, causes SPLUNC1 to adopt an inappropriate conformation, where the ENaC inhibitory domain (also known as the S18 region) cannot bind to ENaC, leading to Na+ hyperabsorption and ASL dehydration. c S18-derived peptides are pH-independent and can inhibit ENaC to reduce Na+ absorption and help normalize airway hydration/mucus clearance in acidic CF airways.
Both CF and COPD airways exhibit increased neutrophil elastase (NE) activity, which has the potential to damage the lung and also to cleave and activate ENaC, exacerbating mucus dehydration and further reducing mucociliary clearance. Alpha-1-antitrypsin (AAT) is an endogenous NE inhibitor which is predicted to improve pulmonary function by blocking NE. Kamada Inc. has an inhaled biological based on human AAT, which is in phase 2 clinical trials for treatment of CF. A potential limitation of AAT is that in addition to NE, several other proteases including cathepsins and metalloproteases are also upregulated in CF/COPD which may also contribute to the lung damage but would not be blocked by AAT.
CF and COPD airways are characterized by high levels of DNA and actin in the lung lumen, which are released by necrotic neutrophils. Excess DNA and actin adversely alter mucus rheology and increase viscosity, leading to decreased mucociliary clearance. Therefore, another approach to increase mucociliary clearance in CF and COPD lungs is to decrease mucus viscosity by cleaving extracellular DNA. Dornase alfa is a recombinant version of human Dnase1 protein that is used as a therapeutic for CF. Dnase1 cleaves extracellular DNA in the lung lumen leading to reduced DNA length/concentration and, therefore, reduced sputum viscosity. Pulmozyme is a recombinant version of human Dnase1 marketed by Genentech for the treatment of CF. Pulmozyme is administered via nebulization and has been shown to reduce the incidence of CF infections.
Biotherapies constitute the fastest growing sector of approved drugs, but their delivery via the lung remains a nascent field. It is increasingly clear, however, that inhaled biological therapeutics can offer some strong advantages over traditional therapeutics including increased potency, reduced systemic availability, and potentially, a longer duration of action. There are several biological drugs that are either approved or in the development pipeline, and here, we have highlighted some that we feel are showing promise to succeed where traditional small molecules and the parenteral delivery route have failed. These examples make it clear that this is an exciting field that warrants future investigation.
Source:
Fellner. R., Terryah. S., Tarran. R., Inhaled protein/peptide-based therapies for respiratory disease 2016, Molecular and Cellular Pediatrics. 3:16