13 Dec Ion Channel Knockdown Improves Obesity Hypoventilation Syndrome in Mice
With the increasing rate of obesity in Australia, obesity hypoventilation syndrome (OHS) and obstructive sleep apnoea (OSA) are on the rise. OHS is characterised by a BMI of 30+ causing sleep disordered breathing and high blood carbon dioxide levels. ~90% of OHS patients present with OSA, with ~70% of cases classed as severe.
For many patients weight loss or CPAP therapy is sufficient to address symptoms but for others this treatment is poorly tolerated or partially effective, creating a demand for alternatives. Scientists at the Polotsky Research Lab at Johns Hopkins Medicine may have discovered a novel solution to this issue, with the identification of ‘TRPM7’ as a key modulator of respiration during sleep.
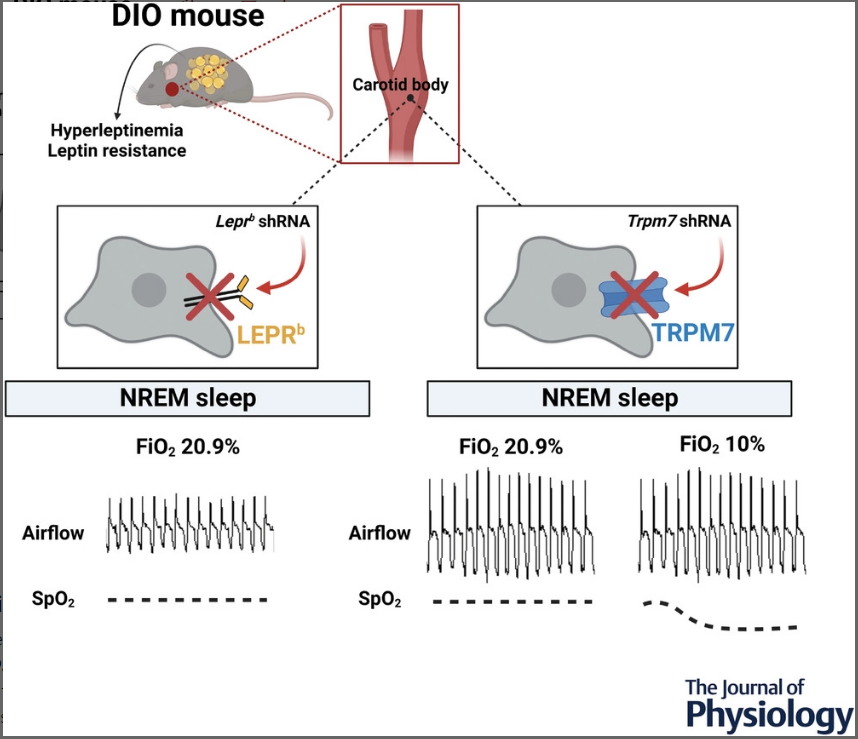
TRPM7 is a positive ion channel located in carotid bodies; specialised blood gas sensors in the neck. These sensors are responsible for detecting and reporting blood gas changes to the central nervous system, resulting in sympathetic changes in heart rate, blood pressure and respiration. When activated, TRPM7 helps deliver sensory information to carotid bodies, opening up to allow positively charged ions to enter the cells and make them more positive. This is usually not problematic but in obesity this response is distorted, with an exaggerated response to low blood oxygen producing hypertension and sleep disordered breathing.
This may be due to the hormone Leptin, which was previously found to increase TRPM7 expression and augment the normal hypoxic response. Leptin is a hormone released by fat so is highly expressed in obese individuals. This is theorised to increase TRPM7 expression on carotid bodies and alter their ability to sense and appropriately respond to blood gas changes.
In earlier studies the group demonstrated this by over expressing the channel to induce hypertension. Male mice with pre-diabetes and obesity (DIO model) were treated with specialised RNA (shRNA) to silence the gene responsible for leptin or TRPM7 expression. They were then implanted with an EEG head mount to measure brain activity and EMG electrodes to measure muscle activity. Respiratory and oxygen saturation measurements were taken manually under normal and low oxygen conditions whilst sleeping. The results showed that the silencing of leptin and TRPM7 did not alleviate the exaggerated chemoreflex in mice during wakefulness but did improve ventilation during sleep. Silencing of leptin alone did not improve sleep disordered breathing, however TRPM7 silencing stimulated normal breathing in the mice. Average minute ventilation during sleep improved by 14% from 0.73 to 0.83 mg/min/L under normal oxygen conditions and by 20% from 1.5 to 1.8 mg/min/L under low oxygen conditions. There were also less obstructive events and a lower average blood pressure witnessed in the TRPM7 knockdown mice.
Unfortunately, the researchers found that despite improved ventilation, oxygen saturation in mice during low oxygen conditions actually decreased with knockdown. This would make potential therapies inaccessible to patients with co-existing respiratory disorders. This discovery is clinically significant as it suggests that pharmacological blockade of TRPM7 would improve both respiration and hypertension in patients with OHS. However, due to the undesirably decreased oxygen saturation in the TRPM7 silenced mice, further research is required.
For more information on the role of TRPM7 in obesity hypoventilation syndrome read an article on the paper by ScienceDaily:
https://www.sciencedaily.com/releases/2022/10/221031091351.htm or read the original paper, published in the Journal of Physiology –
https://physoc.onlinelibrary.wiley.com/doi/10.1113/JP283678
Citation:
Kim, L.J., Shin, M.-K., Pho, H., Tang, W.-Y., Hosamane, N., Anokye-Danso, F., Ahima, R.S., Sham, J.S.K., Pham, L.V. and Polotsky, V.Y. (2022), TRPM7 channels regulate breathing during sleep in obesity by acting peripherally in the carotid bodies. J Physiol, 600: 5145-5162. https://doi.org/10.1113/JP283678

