06 Nov Evaluating Longterm Mandibular Advancement Splint Responders
Background: Mandibular advancement splint (MAS) are an improving therapy for obstructive sleep apnoea (OSA). Evaluating patients who are ‘MAS-responders’ Is important in predicting longterm outcomes, particularly if they are not a candidate for other therapy, including CPAP.
A MAS responder meets the criteria when their apnoea-hypopnoea index (AHI) <10, with a 50% reduction in AHI using the MAS device.
The MAS repositions the lower jaw anteriorly and downwards during sleep. The treatment aims to improve upper airway patency by enlarging upper airway and/or decreasing upper airway collapseability. There are different effects based on the device, either anterior movement of tongue versus anterior movement of mandible.
The best results currently being achieved are with customised titratable 2-piece devices, with vertical extensions holding the lower plate with the advancement provided by lateral ramps on the upper plate.
Titratable custom-made MAS devices have been used in the majority of the efficacy studies. Comparison between device designs indicated that there are only minor differences in treatment effects between custom-made devices, while prefabricated devices are less effective.
MAS therapy have been shown to widen primarily the lateral parts of the upper airway and to reduce pharyngeal collapsibility. MAS treatment reduces the apnoea-hypopnoea index (AHI) compared with placebo in all studies. In a review of 27 studies treatment success with MAS, defined where the AHI was <5, was found in 19–75% of the patients and an AHI of < 10 was reported in 30–94% of the patients.
Promising effects on blood pressure, cardiac function, endothelial function, markers of oxidative stress and simulated driving performance have been reported from MAS.
The Wesley Hospital Sleep Disorders Centre recently presented 7 year data on the efficacy of titratable MAS devices, at the 2017 European Respiratory Society, the world’s largest Thoracic and Sleep conference.
Study Results: MAS construction was initiated after a baseline polysomnographic (PSG) sleep study, with referral to a trained dentist who over-saw the dental evaluation. Advancement of the mandible was aimed for between 50-70% maximum protrusion.
Our protocol included a review PSG study after 3 months of MAS therapy. This was to ensure correct positioning. All patients then had annual surveillance, when defined as a ‘MAS responder’. If the response was suboptimal, further anterior adjustment was made, with the review PSG study performed 3 month later.
118 patients (82 male) were included (mean age 49.8 years, BMI 28.1).
Within 3 months, 65% of patients commenced on MAS therapy met the criteria as MAS responders.
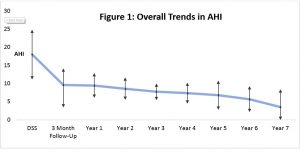
Figure 1: Over 7 years 73% of patients remained MAS responders based upon annual PSG studies and clinical review.
Some required minor MAS adjustments based upon these PSG studies, with a repeat PSG study 3 months later. The variability in AHI increased in later (>5) years.
In a small number of patients, there was a rise in AHI, however a subsequent adjustment in MAS position after 3 months of treatment lead to an effective reduction in AHI .
The variability in AHI is greater after 5 years, and probably represented changing/ evolving risk factors and co-morbidities (usually cardiac / medication related).
Non-responders represented 10% of patients. There was no significant difference in MAS effiacacy between age, sex or BMI.
Figure 2: MAS responders were significant in patients, regardless of OSA severity.
- Average AHI reduction (severe OSA) = 29.6 ± 4.8
- Average AHI reduction (moderate OSA) = 11.7 ± 6.9
- Average AHI reduction (mild OSA) = 4.0 ± 0.1
Overall, all patients with different levels of OSA severity maintained statistically significant benefit utilising annual PSG screening, clinical review, and MAS adjustment if indicated.
Conclusions and recommendations:
This study demonstrates that well constructed titratable MAS devices provide an effective reduction in AHI in longterm responders with mild to severe OSA, in all body positions and during REM.
The increasing variability in AHI, particularly beyond 4-5 years, further favours annual surveillance.
Regular Sleep Physician and Dental reviews, with regular PSG monitoring are essential to long-term success.
We recognise a tendency for patients who do poorly with MAS therapy, and non-responders had a greater rate of ceasing treatment.
The guidelines initiated through this study, are now recognised as ‘best clinical practice’. To obtain the best longterm outcome we recommend therapy be initiated by an experienced medical and dental team, rather than online / pharmacy/ self -made MAS devices.