02 Jun COVID-19 Complications and the ‘Cytokine Release Syndrome’
Clinical presentations vary reflecting direct and indirect tissue damage caused by the ‘cytokine release syndrome (CRS)’. The includes the pathway of introduction with respiratory inflammation, with further damage due to vascular and neurological tissue inflammation, diffuse microangiopathy, small vessel thrombosis and microvascular dysfunction.
The ‘cytokine release syndrome’ (CRS) appears as the principal mechanism for ARDS, multiple-organ dysfunction and death. Therefore for now, the ability to neutralize key inflammatory factors during CRS will be important in reducing morbidity and mortality.

The SARS-CoV-2 infects (mainly) the alveolar epithelial type 2 cells [AEC2] via the AEC2 receptor.
The destruction of epithelial cells plus increase in cell permeability lead to this virus being released. It activates our innate immune system leading to large numbers of cytokines and chemokines being released. Adaptive immunity is also activated.
T cells and B cells play an important antiviral role however they also promote this release of inflammatory cytokines, stimulated by inflammatory factors, producing inflammatory exudates and erythrocytes which enter the alveoli resulting in the first and most important area to be damaged.
Acute Respiratory Failure
Reported in 8% of patients and is the leading cause of mortality in patients. Children can quickly progress to respiratory failure. This varies widely between countries and health services.
Adult Respiratory Distress Syndrome (ARDS)
This has been reported in 15% to 33% in a large case series.


Factors that increase ARDS and death include older age, neutrophilia, elevated lactate dehydrogenase (LDH) levels and elevated D-dimer levels.
Lung transplantation has been reported in China as the sole therapy for end-stage pulmonary fibrosis.
Venous thrombo-embolism (VTE)
COVID-19 related coagulopathy is associated with increased risk of VTE.
It has been reported in 20% to 69% of ICU patients and associated with a poorer prognosis.
Acute pulmonary embolism (PTE) has been reported in 20 to 30% of patients (USA, France).
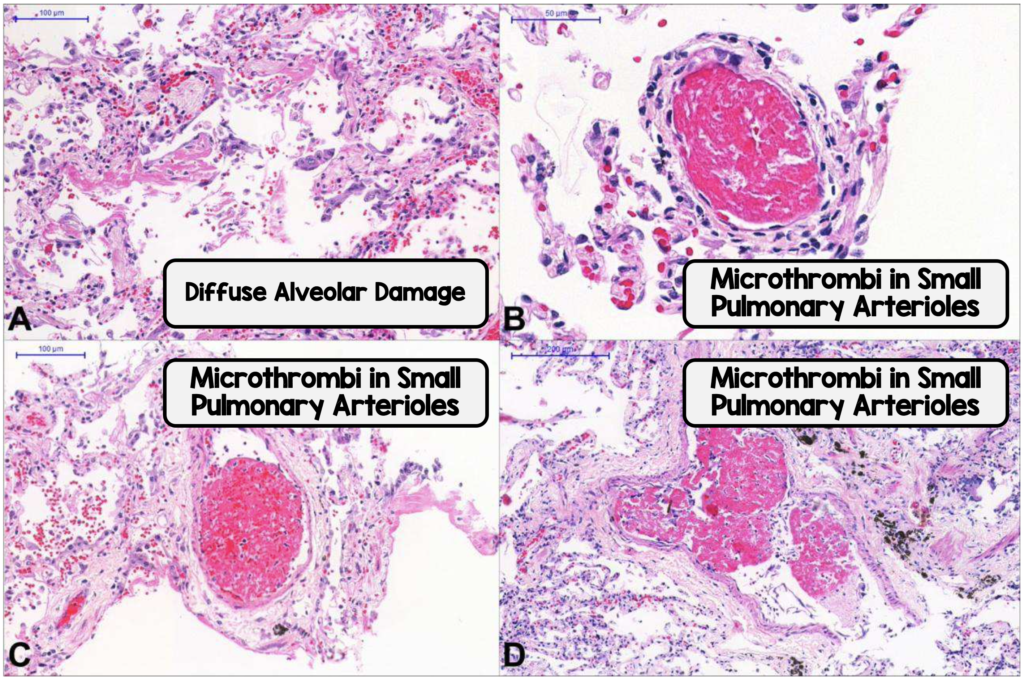
Prophylactic management with both intermediate and full-dose regimens are being studied. The benefit of higher doses is the being able to further reduce undetected thrombi however the bleeding risk appears higher. One study noted 11% of patients at high risk of VTE also had a high risk of bleeding.
Arterial thrombosis with acute limb ischemia cases have been reported.
Cardiovascular Complications
Acute myocardial injury has been reported in 7% to 20%.
Cases of fulminant myocarditis, cardiomyopathy, cardiac tamponade, myopericarditis with systolic dysfunction, pericarditis and pericardial effusion, acute myocardial infarction, cor pulmonale, and takotsubo syndrome have been reported.
Prevalence is higher in those severely or critically ill with longer ICU periods, higher need for ventilation and higher in-hospital mortality.

Acute Kidney Injury
Acute kidney injury has been reported in 3% to 8% of patients.
A large prospective study (>700 patients) noted proteinuria > 40%, haematuria >26%, elevated creatinine and reduced eGFR in 13% to 14%.
Factors increasing risk include age ≥65 years, history of acute kidney injury, chronic kidney disease, heart failure, hepatic disease and diabetes.
Damage appears caused by renal VTE and haemodynamic changes, hypovolemia, direct viral injury to the renal tubules, glomerular pathology and rhabdomyolysis. Direct kidney infection appears rare.
Acute Liver Injury
Acute liver injury with elevated enzymes has been reported in 14% to 53% of patient. These changes occur more commonly with severeillness, however clinically significant liver injury is uncommon.
Viral medications (e.g. lopinavir, ritonavir) may also have a detrimental effect on liver injury.
Mild pancreatic injury has been reported in 17% of patients and associated with elevated serum amylase or lipase levels. It is unknown if this reflects direct viral effect or the immune response.
Neurological Complications
The SARS-CoV-2 virus exhibit particular neurotropic properties. Complications therefore result from direct CNS invasion, with the virus detected within brain tissue and the CSF.
Neurologic symptoms have been noted in 36% of patients in a larger series (214 patients), and more commonly with severe illness.
Reports of sudden loss of smell or taste (anosmia, dysgeusia) suggest a neuronal pathway for this neurotropic virus to enter the CNS by infecting sensory or motor nerve endings, achieving retrograde (or anterograde) neuronal transport.
Complications include viral encephalitis, meningitis, acute cerebrovascular compromise complicated by impaired consciousness, ataxia, seizures, acute neuralgia, skeletal muscle injury, corticospinal tract signs and infectious toxic encephalopathy. These patients have a poor prognosis.

Other syndromes being reported included large-vessel stroke in a small number of patients (<50 years of age, New York) plus acute Guillain-Barre syndrome in one patient (Iran).
Cytokine Release Syndrome
The ‘cytokine release syndrome’ causes elevated serum proinflammatory cytokines e.g. tumor necrosis factor (TNF) alpha, interleukin-2, interleukin-6, interleukin-8, interleukin-10, granulocyte-colony stimulating factor (GCSF), monocyte chemoattractant protein-1 plus inflammatory markers e.g. CRP, serum ferritin.
Interleukin-6 has been particularly associated with severe COVID-19 and increased mortality.
Anti-inflammatory and immunosuppressive treatments are being trialled eg tocilizumab, Janus kinase inhibitors, anakinra (biological DMARD).
Paediatric Multisytem Inflammatory Syndrome
A small number of children (USA, UK) have developed a significant systemic inflammatory response.
The syndrome shares features with Kawasaki disease, toxic shock syndrome and bacterial meningitis. There is a cautionary note this may be another as yet unidentified infectious pathogen.
Septic Shock
Reported in 4% to 8% of patients in case series.
Management in critically ill patients is including conservative fluid strategy with a vasoactive agent eg noradrenaline. adrenaline, vasopressin, dopamine and dobutamine. Low-dose corticosteroid therapy is recommended for refractory shock.
Disseminated Intravascular Coagulation (DIC)
Reported in 71% of non-survivors.
DIC is a manifestation of coagulation failure and a link in the development of multi-organ failure. Patients may be at high risk of bleeding/hemorrhage and venous thromboembolism. Rhabdomyolysis is reported as a late complication.
Markers include elevated fibrinogen, elevated D-dimer, minimal change in prothrombin time, partial thromboplastin time, and platelet count in the early stages of infection. Increasing interleukin-6 levels correlate with increasing fibrinogen levels.
Pregnancy related Complications
Retrospective reviews of pregnant women found they have fewer adverse maternal and neonatal complications and outcomes than expected for SARS or MERS.
Foetal /neonatal adverse outcomes included perinatal death, preterm birth, neonatal death, foetal distress, premature labor, respiratory distress, thrombocytopenia and abnormal liver function.
Maternal adverse outcomes include death and miscarriage, including during the second trimester.
Secondary Infections
Most admitted patients (est >71%) receive antibiotics however no information is available on antimicrobial sensitivities of any organisms identified or type and duration of antimicrobial treatment.
COPD is a risk factor for severe COVID-19 disease with many patients having chroni bacterial infections before the SARS-CoV-2 infection.
Limited reports suggest secondary infections occur in 6% to 10% patients, however some centres have noted 50% of patients with COVID-19 who died had secondary bacterial infections.
More data on co-infections is now being acquired and shared to establish their importance with COVID-19 severity and mortality.

